Abstract
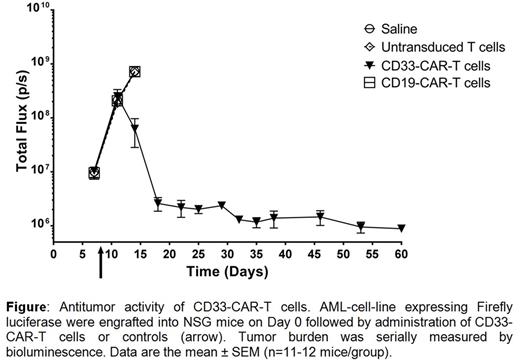
Relapsed/refractory acute myeloid leukemia (AML) is an aggressive malignancy with poor outcomes underscoring the need to implement new therapies. Adoptive transfer of genetically modified T cells with specificity redirected through a chimeric antigen receptor (CAR) has resulted in clinical responses, particularly for B-cell malignancies. CD33 is an attractive target for immunotherapy of AML as this transmembrane protein is expressed on majority of AML blasts. On target, but off tumor effects are anticipated as this target is also expressed on normal myeloid cells and on a subset of activated T and NK cells. Therefore, we generated a lentiviral vector co-expressing a CD33-specific CAR with a kill switch, HER1t, for the generation of CD33-specific CAR+HER1t+ T (CD33-CAR-T) cells. HER1t improves the safety profile by providing a mechanism for selective in vivo depletion of these genetically modified T cells upon addition of the clinically-available monoclonal antibody, cetuximab. Co-expression of CAR and HER1t on transduced T cells was confirmed by flow cytometry and western blot analyses. The redirected specificity was demonstrated by the specific killing of CD33+ tumor cells as well as significant release of cytokines IFNγ, TNFα, IP-10, IL-13, IL-18, and LIF upon co-culture with AML tumor cells. The ability to eliminate AML in vivo was determined in immunocompromised (NSG) mice bearing MOLM-13 (CD33+CD19neg) tumor. A single administration of human CD33-CAR-T cells resulted in a significant reduction in tumor burden (Figure) and improvement in overall survival with an overall median survival time of 46 days compared to 15 days for control treated mice (saline only, untransduced (CARneg) T cells, or CD19-specific CAR+ T (CD19-CAR-T) cells). Assessment from the plasma of CD33-CAR-T treated mice revealed production of cytokines consistent with observations from in vitro activation of genetically modified T cells. CD33-CAR-T cells were effectively eliminated in the presence of cetuximab both in vitro in an antibody dependent cell cytotoxicity assay and in an in vivo adoptive T cell transfer model in immunocompromised NOD/SCID mice. In preparation for a clinical trial, we manufactured CD33-CAR-T cells from an individual with relapsed AML. As with T cells from healthy volunteers, transduced T cells from the patient co-expressed CAR and HER1t and were effective at specifically eliminating autologous CD33+ tumor as well as producing cytokines in response to CAR signaling. These pre-clinical data provide a strong rationale to evaluate CD33-CAR-T therapy in a clinical trial for treatment of AML. The FDA has approved an investigational new drug application to initiate phase 1 clinical trial at MD Anderson Cancer Center for treatment of CD33+relapsed/refractory AML with autologous CD33-CAR-T cells.
Song: Intrexon Corporation: Employment. Tian: Intrexon Corporation: Employment. Carvajal-Borda: Intrexon Corporation: Employment. Plummer: Intrexon Corporation: Employment. Shah: Intrexon Corporation: Employment. Wierda: Emergent: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Karyopharm: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Kite: Research Funding; Genzyme: Consultancy, Honoraria; The University of Texas MD Anderson Cancer Center: Employment; Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Sanofi: Consultancy, Honoraria; Acerta: Research Funding; Juno: Research Funding. Cooper: Organovo Holdings: Equity Ownership; Sangamo Biosciences: Patents & Royalties; Miltenyi Biotec: Honoraria; Ampliphi Biosciences: Equity Ownership; Argos Therapeutics: Equity Ownership; Intrexon Corporation: Equity Ownership, Patents & Royalties; Procter & Gamble: Equity Ownership; Targazyme, Inc.: Equity Ownership; Ferring: Consultancy; Immatics: Equity Ownership, Patents & Royalties, Research Funding; Ziopharm: Employment, Equity Ownership, Research Funding. Chan: Intrexon Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal